Abstract
Therapy-related acute myelogenous leukemia (t-AML) is associated with adverse genetic lesions, complex karyotype, and TP53 mutation; it is challenging to treat and confers a poor prognosis. We describe a 74-year-old female patient with AML that developed 9 years after receiving 6 cycles of cytotoxic (FOLFOX) chemotherapy as adjuvant treatment of colorectal carcinoma. At diagnosis, the bone marrow (BM) biopsy revealed blast count of 35%, with normal karyotype, 5q loss and AML1 (RUNX1) locus (21q22) amplification by fluorescence in situ hybridization (FISH). Initial treatment with 5 cycles of azacitidine (AZA) failed to induce a response. The patient was subsequently treated on a Phase 1 trial of flotetuzumab (MGD006/S80880) (FLZ), a novel T-cell redirecting (CD123 x CD3) DART protein [NCT02152956]. Prior to FLZ, BM blasts demonstrated clonal evolution with a complex karyotype (92,XXXX, t(14;21)(q22;q22)) and new IDH1, and TET2 mutations.
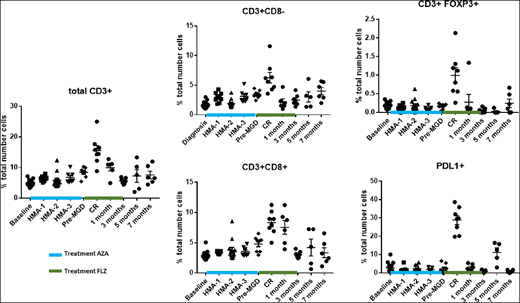
Patient received a total of 3 cycles (28 days/cycle) of FLZ. After one FLZ cycle, the patient achieved a (complete response) CR, with normal cytogenetics, and residual IDH1 and TET2 mutations. After 2 additional cycles of FLZ consolidation, the CR was maintained, with loss of the IDH1 mutation, but persistence of the TET2 mutation. CR was maintained with no additional therapy for approximately 7 months. Serial BM samples were evaluated for T cells (CD3, CD4, CD8, FOXP3 and PD-L1) using multiplex IHC (Fig 1). BM T cells were unchanged during AZA treatment. At the initial bone marrow on FLZ (CR), significant increases in CD3+, CD3+CD8-, CD3+ CD8+, and FOXP3-positive T cells and PD-L1 expression compared to baseline were noted. CD3+CD8+ T cells persisted in the BM 1 month beyond completion of the additional 2 cycles of consolidation with FLZ, while other T cells subsets and PD-L1 expression returned to baseline (Fig. 1) Three months after the last FLZ treatment, all T cell subsets had returned to baseline, with an early increase in leukemic blasts (8%), but normal cytogenetics. Five months after FLZ treatment blasts were unchanged, but a new abnormal cytogenetic clone, with additional mutations, accompanied by a rise in PD-L1 expression cells was observed. Seven months after FLZ treatment, frank leukemia with 50% blasts developed, and all T cell subsets and PD-L1 expression had returned to pre-treatment levels.
Consistent with its proposed mechanism of action, these data highlight for the first time the induction of an increase in T-cell infiltration, which persist beyond FLZ, in the bone marrow microenvironment of an AML patient that also achieved a complete and durable anti-leukemic response after treatment with FLZ.
Lelièvre:Servier: Employment. Wigginton:MacroGenics: Employment. Davidson-Moncada:MacroGenics: Employment. Fox:MacroGenics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal